Stem Cells as an Ideal Carrier for Gene Therapy, Stem cells have captivated the scientific community and public imagination for their potential to revolutionize medicine. Among their many promising applications, their role as carriers for gene therapy stands out, offering hope for treating a myriad of genetic disorders. As we progress through 2024, the integration of stem cells into gene therapy has seen significant advancements, driven by cutting-edge research and technological innovations. This article explores why stem cells are considered ideal carriers for gene therapy and examines the latest developments that underscore their potential in this transformative field.
Read More: AI Content Writing Tools in 2024
Understanding Stem Cells and Gene Therapy
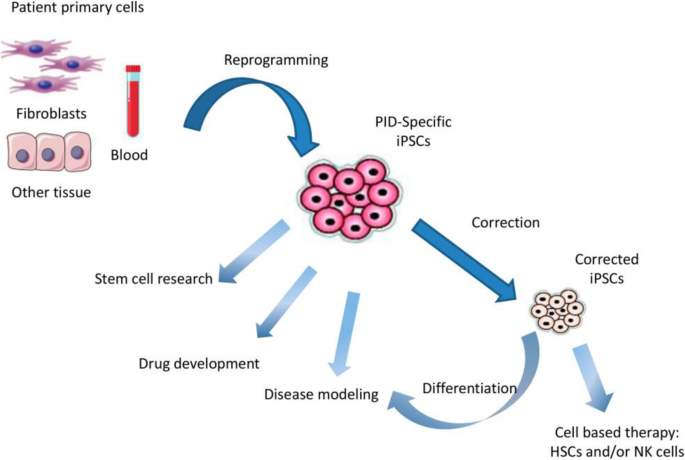
Stem Cells: A Primer
Stem cells are unique in their ability to differentiate into various cell types and self-renew, making them indispensable in developmental biology and regenerative medicine. They are broadly categorized into embryonic stem cells (ESCs), which are pluripotent and can give rise to virtually any cell type, and adult stem cells, which are multipotent and more restricted in their differentiation potential. Induced pluripotent stem cells (iPSCs), generated by reprogramming somatic cells, offer a versatile and ethically favorable alternative to ESCs.
Gene Therapy: A New Frontier
Gene therapy involves the introduction, removal, or alteration of genetic material within a patient’s cells to treat or prevent disease. By targeting the genetic root of disorders, gene therapy holds the promise of providing lasting and potentially curative treatments for conditions that are currently managed with symptomatic therapies.
The Synergy of Stem Cells and Gene Therapy
One of the primary reasons stem cells are ideal carriers for gene therapy is their inherent ability to home to specific tissues and integrate into the host genome. This makes them exceptional vehicles for delivering therapeutic genes to precise locations within the body. For instance, hematopoietic stem cells (HSCs) can be used to deliver genes to the blood and immune system, offering a potential cure for genetic blood disorders such as sickle cell anemia and beta-thalassemia.
Sustained Gene Expression
Stem cells’ capacity for self-renewal ensures that therapeutic genes introduced via these cells can provide long-term expression. This sustained gene expression is crucial for chronic conditions, where continuous production of a therapeutic protein is necessary for disease management. By integrating the therapeutic gene into the stem cell genome, the therapy can potentially persist for the patient’s lifetime, reducing the need for repeated treatments.
Reduced Immune Response
Gene therapy often faces the challenge of immune rejection, where the body recognizes the introduced genetic material as foreign and mounts an immune response. Stem cells, particularly iPSCs derived from the patient’s own cells, can minimize this risk. By using autologous stem cells (cells derived from the patient), the likelihood of immune rejection is significantly reduced, enhancing the safety and efficacy of the treatment.
Advances in Stem Cell-Based Gene Therapy in 2024
CRISPR-Cas9 and Genome Editing
The advent of CRISPR-Cas9 technology has revolutionized gene therapy by providing a precise and efficient tool for genome editing. In 2024, CRISPR-Cas9 has been extensively integrated into stem cell-based therapies to correct genetic mutations at their source. Researchers have successfully used CRISPR-Cas9 to edit iPSCs, correcting mutations responsible for conditions such as cystic fibrosis and muscular dystrophy. These corrected cells can then be differentiated into the relevant cell types and reintroduced into the patient, offering a potential cure.
Organoids and Disease Modeling
Stem cell-derived organoids, three-dimensional structures that mimic real organs, have become invaluable tools for studying diseases and testing gene therapies. In 2024, advancements in organoid technology have enabled researchers to create more accurate models of human organs, allowing for better understanding of disease mechanisms and more precise testing of gene therapies. For example, liver organoids derived from iPSCs have been used to model genetic liver diseases and test the efficacy of gene-editing approaches, paving the way for personalized and effective treatments.
Combination Therapies
Combining stem cell therapy with other treatment modalities has shown promising results. For instance, researchers are exploring the synergistic effects of combining stem cell-based gene therapy with immunotherapy for cancer treatment. Engineered stem cells can deliver genes that not only correct genetic defects but also enhance the immune system’s ability to target and destroy cancer cells. This multi-faceted approach holds the potential to improve outcomes for patients with complex diseases such as cancer.
Bioengineering and Scaffold Technologies
In 2024, bioengineering and scaffold technologies have advanced to support the growth and differentiation of stem cells in three-dimensional environments, closely mimicking the natural tissue architecture. These scaffolds can be impregnated with therapeutic genes, allowing for localized and sustained gene delivery. This approach has shown promise in regenerative medicine, where engineered tissues can be used to repair damaged organs, such as the heart or cartilage, with integrated gene therapy to promote healing and prevent disease recurrence.
Clinical Applications and Success Stories
Hemoglobinopathies
One of the most significant success stories in stem cell-based gene therapy is the treatment of hemoglobinopathies, such as sickle cell anemia and beta-thalassemia. In 2024, clinical trials have demonstrated the efficacy of using gene-edited HSCs to correct the genetic defects underlying these disorders. Patients treated with their own gene-corrected HSCs have shown remarkable improvements, with some achieving complete remission. This approach not only alleviates the symptoms but also addresses the root cause of the disease, offering a potential cure.
Inherited Retinal Diseases
Inherited retinal diseases, which lead to progressive vision loss and blindness, have also seen promising advancements with stem cell-based gene therapy. Researchers have developed protocols to generate retinal cells from patient-derived iPSCs and correct the genetic mutations causing these conditions. Early clinical trials have shown that these gene-corrected retinal cells can integrate into the retina and restore some degree of vision, offering hope to patients who previously had no effective treatment options.
Neurodegenerative Diseases
Stem cell-based gene therapy is making strides in the treatment of neurodegenerative diseases such as Parkinson’s and Huntington’s disease. In 2024, clinical trials are exploring the use of gene-edited neural stem cells to replace damaged neurons and deliver therapeutic genes directly to the brain. These trials aim to halt or slow the progression of these debilitating diseases, providing patients with improved quality of life and increased longevity.
Challenges and Future Directions
Ethical and Regulatory Considerations
Despite the promising advancements, stem cell-based gene therapy faces several ethical and regulatory challenges. The use of CRISPR-Cas9 and other gene-editing technologies raises concerns about off-target effects and the potential for unintended genetic modifications. Regulatory agencies are tasked with ensuring the safety and efficacy of these therapies, necessitating rigorous testing and long-term follow-up studies.
Scalability and Manufacturing
The scalability and manufacturing of stem cell-based gene therapies present significant challenges. Producing high-quality, gene-edited stem cells in sufficient quantities for widespread clinical use requires advanced biomanufacturing techniques and stringent quality control measures. Researchers are working to develop scalable processes and automated systems to meet the growing demand for these therapies.
Access and Affordability
Ensuring access to and affordability of stem cell-based gene therapies is another critical challenge. These therapies are often expensive and resource-intensive, limiting their availability to a broader patient population. Policymakers, healthcare providers, and industry stakeholders must collaborate to develop strategies that make these groundbreaking treatments accessible to all who need them.
Conclusion
As we navigate through 2024, stem cells continue to demonstrate their immense potential as carriers for gene therapy, offering hope for treating a wide range of genetic disorders. The synergy between stem cell biology and gene-editing technologies like CRISPR-Cas9 has ushered in a new era of precision medicine, where therapies can be tailored to individual patients with unprecedented accuracy. While challenges remain, the rapid advancements in this field hold the promise of transforming healthcare and improving the lives of millions worldwide. The future of stem cell-based gene therapy is bright, and continued research and innovation will undoubtedly unlock even greater possibilities in the years to come.
FAQs: Stem Cells as an Ideal Carrier for Gene Therapy in 2024
1. What are stem cells and why are they important?
Stem cells are unique cells with the ability to differentiate into various cell types and self-renew. They are crucial for their roles in development, tissue repair, and regenerative medicine. Their versatility makes them ideal for applications in gene therapy, where they can deliver therapeutic genes to specific tissues and provide sustained gene expression.
2. What is gene therapy?
Gene therapy involves modifying or introducing genetic material into a person’s cells to treat or prevent disease. It aims to address the root cause of genetic disorders by correcting faulty genes or providing new genes to help fight diseases.
3. Why are stem cells considered ideal carriers for gene therapy?
Stem cells are ideal carriers for gene therapy due to their ability to target specific tissues, integrate into the host genome, provide sustained gene expression, and reduce the risk of immune rejection, especially when using autologous (patient-derived) stem cells.
4. How has CRISPR-Cas9 impacted stem cell-based gene therapy?
CRISPR-Cas9 has revolutionized gene therapy by enabling precise and efficient genome editing. In stem cell-based gene therapy, it allows for the correction of genetic mutations in stem cells, which can then be used to generate healthy tissues or organs for transplantation back into patients.
5. What are organoids, and how are they used in gene therapy?
Organoids are three-dimensional structures derived from stem cells that mimic real organs. They are used in gene therapy to model diseases and test gene-editing techniques, providing a better understanding of disease mechanisms and enabling more accurate testing of therapeutic approaches.
6. Can you give examples of successful stem cell-based gene therapies?
Some success stories include the treatment of hemoglobinopathies like sickle cell anemia and beta-thalassemia, where gene-edited hematopoietic stem cells have led to significant improvements and potential cures. Another example is the use of gene-corrected retinal cells to restore vision in patients with inherited retinal diseases.
7. What challenges does stem cell-based gene therapy face?
Challenges include ethical and regulatory considerations, the risk of off-target effects from gene editing, scalability and manufacturing issues, and ensuring access and affordability for patients.
8. How are ethical concerns addressed in stem cell-based gene therapy?
Ethical concerns are addressed through rigorous regulatory frameworks that ensure the safety and efficacy of gene-editing technologies. Long-term follow-up studies and strict testing protocols are implemented to monitor and mitigate potential risks.
9. What are the future directions for stem cell-based gene therapy?
Future directions include improving gene-editing techniques for greater precision, developing scalable manufacturing processes, enhancing the integration of stem cell and gene therapy with other treatment modalities, and ensuring broader access to these therapies through policy and healthcare collaborations.
10. How do stem cell-based gene therapies minimize immune rejection?
By using autologous stem cells—stem cells derived from the patient—stem cell-based gene therapies significantly reduce the risk of immune rejection, as the patient’s immune system is less likely to recognize the cells as foreign.
11. What role do bioengineering and scaffold technologies play in this field?
Bioengineering and scaffold technologies support the growth and differentiation of stem cells in three-dimensional environments, closely mimicking natural tissue structures. These scaffolds can be used for localized and sustained delivery of therapeutic genes, enhancing the effectiveness of regenerative treatments.
12. Are there any ongoing clinical trials in 2024 for stem cell-based gene therapy?
Yes, several clinical trials are ongoing in 2024. These include trials for treating genetic blood disorders, inherited retinal diseases, and neurodegenerative diseases using gene-edited stem cells and other advanced therapeutic approaches.
13. How can patients access stem cell-based gene therapies?
Access to these therapies typically involves participation in clinical trials or treatments offered at specialized medical centers. Patients should consult with healthcare providers and explore available options, including seeking information from research institutions and clinical trial registries.
14. What is the potential impact of stem cell-based gene therapy on healthcare?
Stem cell-based gene therapy has the potential to transform healthcare by providing curative treatments for genetic disorders, reducing the burden of chronic diseases, and improving the quality of life for patients with previously untreatable conditions.
15. How can the scalability of stem cell-based gene therapies be improved?
Researchers are working on developing advanced biomanufacturing techniques and automated systems to produce high-quality, gene-edited stem cells at scale. This involves improving culture conditions, optimizing differentiation protocols, and ensuring stringent quality control measures.